As is known, temperature fluctuation can cause a remarkable change in the polymer permeability. Why should this happen? That is mainly determined by material structure on one hand and by the property of permeability on the other.
1. Structure characteristic of the high polymer
According to its molecular arrangement, the solid high polymer can be divided into crystallized, noncrystalline and orientative polymer. Most of the crystallized polymer is hemicrystalline with both crystallized and noncrystalline parts, in which the only difference is its degree of crystallization.
Crystallized part of the polymer is theoretically thought as an area that cannot be permeated during the diffusion of infiltration molecules in polymer. It is the noncrystalline part that the diffusion takes place and most of the “ ‘macro' diffusion model” is built in connection with the noncrystalline polymer. One of the most popular molecule statistical models describing the diffusion of simple infiltration in noncrystialline rubber-like polymer is the molecule model of Pace and Datyner. According to this model, the molecule of infiltration can pass through polymer matrix in ways of " vertical movement " and " horizontal movement ". " vertical movement " refers to movement of infiltration molecule along an axis direction of the channel formed by adjoining and parallel molecule chain while the " vertical movement " refers to movement of infiltration molecule vertical to an axis direction of the channel formed by adjoining and parallel molecule chain. The model of flexible volume is also a kind of popular diffusion model. In this model, flexible volume of the polymer is considered to be the " empty volume " within the polymer molecule chains. It is supposed that molecule chain of polymer and the movement of infiltration molecule are mainly determined by the available flexible volume within the polymer-infiltration system.
The longer the polymer molecule chain, the more its conformation. When temperature is increased, conformation changing of molecule chain will be accelerated as a result of thermal motion and this in turn will decrease the extent of polymerization. For the molecule model of Pace and Datyner, it can be explained that a broadening of the former mentioned parallel channel caused by temperature rising will accelerate the “ horizontal movement ” of infiltration molecule. At the same time, the accelerating of conformation change will increase the distance among two adjoining molecule chain as a result of which “vertical movement” will also be speeded up. As for the model of flexible volume, the explanation is that when temperature rises, the flexible volume available for infiltration molecule to permeate the polymer is increased. Then the diffusion of infiltration molecule in the polymer is accelerated. That is to say, a rising in temperature will reduce the permeability of material.
2. Characteristic of the gas molecule movement
Routine permeability testing uses common inorganic gas as infiltration material. Since gas has a nature of diffusibility and compressibility, the state of a specific amount of gas can be described with three parameters of pressure, volume, and temperature. Under normal temperature and pressure, the molecule's size is much smaller comparing with the average distance between molecules. It can be considered as ideal gas when the size of molecule is ignored. Equation for ideal gas is:
pV = nRT
Internal energy of ideal gas:
E=i/2 *nRT
In the equation :
E —— Internal energy of ideal gas
i —— liberty index of gas molecules
n —— mol
R —— constant of mol , 8.31J/mol · K
T —— thermodynamics temperature
For specific gas, i , n are fixed value and the internal energy of ideal gas is only the function of temperature. In this function, the internal energy of ideal gas is in direct proportion to T. This result is approximately the same with that of the experiment performed under a condition similar to the room temperature. Under the normal temperature and pressure, the higher the gas temperature is, the more intensive the thermal motion of gas molecule and the greater the energy will be. When gas diffuses as infiltration material within polymer, a rise in temperature will increase the energy of gas molecule. This can help gas molecule to reach the energy value needed to diffuse among molecule chains. In this way, diffusion coefficient of gas molecule in the polymer will increase and the permeability of the material will be reduced.
3. Arrhenius relation
The influence of temperature fluctuation on the permeability of inorganic gas in polymer is very obvious. A rise in temperature will increase P , D , S. Its relation with temperature can be demonstrated by Arrhenius equation.

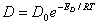
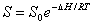
The influence of temperature fluctuation on P ,D ,S is different if the polymer and infiltration gas are different . Though there is difference existing in the corresponding value of P0 ,D0 ,S0 and EP ,ED ,△H , they are all found to be in accordance with the Arrhenius equation. The data fitting available in Labthink VAC-V1 can be used to get specific sample as well as the P0 ,D0 ,S0 and EP,ED ,△H value of infiltration gas. When providing fits to PC film① and another unknown film A② with the permeability coefficient of oxygen at 30 °C, 35 °C, 40 °C , We can get the following data of fitting: PC film,P0 =9305.716509507,EP =16822.675460039;film A,P0=742581.566783723, EP=36973.239405092 。From 30 °C to 40 °C , increasing extent of the oxygen permeability coefficient of film A is much larger than that of PC film.
Note:
①for test data and fit curve of PC, please refer to《 Film Permeability under Specific Temperature-Permeability Coefficient Fitting 》 at Labthink Forum.
② film A is offered by Germany Mecadi Lab. For detailed testing data see section 4.
4. Permeability testing of film A under different temperature
Film A is unknown in material and it is 80 μ m in thickness. Labthink VAC-V1 is used to perform the oxygen permeability testing under the temperature of 23 °C, 30 °C, 35 °C, 40 °C, 45 °C . For testing data, see form 1.]
Form 1. Measured Data of Film A ①
| Temperature (°C) | O2 Infiltration amount (cm3 /m2· 24h · 0.1MPa ) | O2 Permeability ( 10 -11 cm3 · cm/cm2· s · cmHg ) |
|---|---|---|
| 23 | 19.17 | 0.2335 |
| 30 | 25.862 | 0.3150 |
| 35 | 32.447 | 0.3953 |
| 40 | 41.321 | 0.5034 |
| 45 | 50.703 | 0.6177 |
Note :① Form 1 does not contain all the testing data , detailed data please refer to the week data online updated several weeks ago.
If we provide fits to experimental data under 23 °C and 30 °C in form 1, the oxygen permeability we get at 35 °C is 31.754cm3/m2 · 24h · 0.1Mpa , oxygen permeability at 40 °C O2 is 38.744cm3/m2 · 24h · 0.1Mpa and oxygen permeability at is 45 °C 46.978cm3/m2 · 24h · 0.1Mpa. These results are very close to the real testing results listing in form 1. If we use the experimental data under 23 °C, 30 °C, 35 °C, 40 °C to fit the oxygen permeability at 45 °C , the result we get is 50.234 cm3/m2 · 24h · 0.1Mpa, which differs only 0.925% from the testing result. The above results prove that the more the fitting data, the closer the fitting result will be with the testing result. Since the material of film A is unknown, it can be inferred there is no selectivity for material in the fitting.
Putting the fitting data of oxygen infiltration amount of film A under the temperature between -100 °C to 150 °C( 173 ~ 423K ) in Excel, we got graph 1 .From this graph we can see that the infiltration amount of film A begin to increase obviously between 50 ~ 60 °C and its increase gradually accelerated as the temperature is rising. The infiltration amount reaches about 400cm3/m2 · 24h · 0.1Mpa at a temperature of 100 °C with certain permeability maintaining .
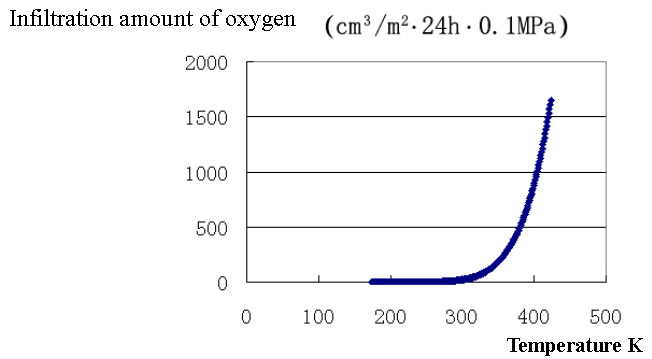
Graph 1. Oxygen Infiltration Amount and Temperature Curve for Film A
5. Conclusion
To sum up, the fluctuation of temperature has a significant influence on the permeability of material. That is why the scope of temperature under which a material is used is an important index of choosing wrappings. If temperature goes beyond this scope of application, it will directly influence the property of material. For the permeability testing under specific temperature, using data fitting is one of the best choices. It is a way not only convenient and timesaving, but also scientific and reliable.
